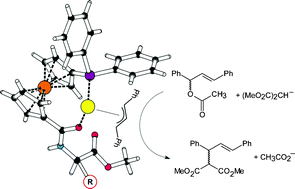
An extensive series of chiral amino acid amides prepared from 1′-(diphenylphosphino)ferrocene-1-carboxylic acid (Hdpf) or its planar-chiral isomer, 2-(diphenylphosphino)ferrocene-1-carboxylic acid, have been tested as ligands for Pd-catalysed asymmetric allylic substitution reactions. In alkylation of 1,3-diphenylallyl acetate as a model substrate with dimethyl malonate the ligands performed well in terms of both reaction rate and enantioselectivity, achieving up to 98% ee. In contrast, the reactions of the same substrate with other nucleophiles proceeded either slowly and with poor ee's (amination with benzylamine) or not at all (etherification with benzyl alcohol). In order to rationalise the influence of the ligand structure on the reaction course, three model complexes, viz. [(η3-methallyl)PdCl(L-κP)], [(η3-methallyl)Pd(L-κ2O,P)]ClO4 and [(η3-methallyl)Pd(L-κP)2]ClO4 have been prepared from the achiral amide Ph2PfcCONHCH2CO2Me (L; fc = ferrocene-1,1′-diyl) and structurally characterised. The coordination study showed that the amido-phosphines readily form 1 : 1 complexes as O,P-chelates where the amino acid chirality is brought close to the Pd atom. At higher ligand-to-metal ratios, however, simple P-monodentate coordination prevails, minimising the influence of the chiral amino acid pendant.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...