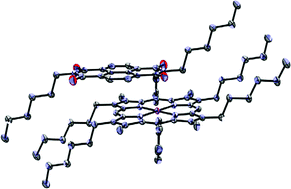
New donor–acceptor hybrids of Zn(II)-metallated 5,15-diaryl porphyrins have been designed and synthesised via the porphyrin interactions with an electron acceptor molecule, di-n-hexylN-substituted 1,2,4,8-naphthalenetetracarboxylic diimide (NDI). Binding interactions within these supramolecular complexes were investigated in the solid state by synchrotron X-ray diffraction and probed in solution by 1H NMR spectroscopy. The systematic modulation of the porphyrin π-density was achieved, for the first time as multiple methoxy and fluorine groups were introduced as substituents to the 5,15-diaryls of the porphyrin. For these, the variation of the porphyrin–NDI binding strengths determined by 1H NMR titrations was shown, using the Swain's type dual parameter approach, to be closely linked with the peripheral substitution pattern of the diaryl porphyrins validated by crystallography. The new 1 : 1 donor–acceptor complexes formed display characteristic features of the aromatic-stacked systems, i.e. the parallel arrangement and short interplanar separation between the substituted porphyrin and NDI. Synthetic modification of electron-density on the porphyrin surface by introducing substituents at peripheral sites of functionalised porphyrins represent a general solution towards electronically tunable aromatic surfaces: an understanding of their solution and solid state behaviour will significantly improve the rational design of new functional donor–acceptor supramolecular materials with potential applications ranging from new energy materials to dye-sensitised solar cells, photovoltaics and future drug delivery devices.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...