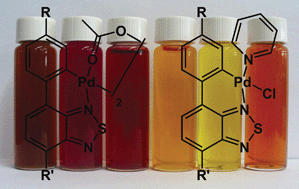
The cyclopalladation of the 4-aryl-2,1,3-benzothiadiazoles 1a–c with palladium acetate in acetic acid afforded the novel dimeric complexes 2a–c in good yields. These were then converted into the monomeric pyridine-, chloro-coordinated cyclometallated complexes 3a–c through reaction with lithium chloride in acetone and then pyridine in dichloromethane. All complexes were fully characterized by means of NMR, IR and elemental analysis. The X-ray structure of complex 2c revealed that it presents transoid geometry, whereas the X-ray structure of 3c shows that the pyridine ligand and the thiazole ring are mutually trans. Photophysical properties were investigated by means of UV-Vis absorption and fluorescence emission in solution. Solid-state diffuse reflectance UV-Vis spectra (DRUV) were also applied in order to better characterize the complexes photophysics in the solid state. All complexes present intense absorption at around 300 nm (λ1) via1LC transitions located in BTD ligands, and additional low energy absorption bands, higher than 450 nm (λ2) of 1MLCT character. The complexes are fluorescent in solution at room temperature, where two emission bands could be observed, a high energy band (excitation @ λ1) ascribed to the ligand emission and an additional red shifted low intense band (excitation @ λ2) due to the complex emission.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...