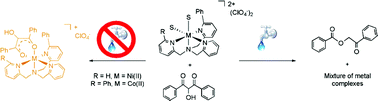
Reaction conditions were evaluated for the preparation of [(6-PhTPA)Ni(PhC(O)C(OH)C(O)Ph)]ClO4 (3) and [(6-Ph2TPA)Co(PhC(O)C(OH)C(O)Ph)]ClO4 (7), two complexes of structural relevance to the enzyme/substrate (ES) adduct in Ni(II)- and Co(II)-containing forms of acireductone dioxygenase. The presence of water in reactions directed at the preparation of 3 and 7 was found to result in isomerization of the enolate precursor 2-hydroxy-1,3-diphenylpropane-1,3-dione to give the ester 2-oxo-2-phenylethylbenzoate. Performing synthetic procedures under dryer conditions reduced the amount of ester production and enabled the isolation of 3 in high yield. This complex was comprehensively characterized, including by X-ray crystallography. Using similar conditions for the 6-Ph2TPACo-containing system, the amount of ester generated was only modestly affected, but the formation of a benzoate complex ([(6-Ph2TPA)Co(O2CPh)]ClO4, 10) resulting from ester hydrolysis was prevented. The best preparation of 7 was found to involve dry conditions and short reaction times. The approach outlined herein toward determining appropriate reaction conditions for the preparation of 3 and 7 involved the preparation and characterization of several air-stable (6-PhTPA)Ni- and (6-Ph2TPA)Co-containing analog complexes having enolate, solvent, and benzoate ligands. These complexes were used as paramagnetic 1H NMR standards for evaluation of reaction mixtures containing 3 and 7.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...