Efficient and stereoselective syntheses of isomeric trifluoromethyl-platinum(iv) chlorides†
Abstract
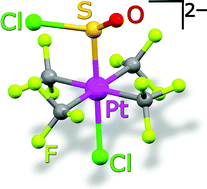
The homoleptic, square-planar trifluoromethylplatinate(II) compound [NBu4]2[Pt(CF3)4] (1) reacts with SOCl2 undergoing oxidative addition of a S–Cl bond to give the octahedral species [NBu4]2[trans-Pt(CF3)4Cl(SOCl)] (4), that contains the unusual chlorosulfinyl
- This article is part of the themed collection: Dalton Transactions 40th Anniversary

 Please wait while we load your content...
Please wait while we load your content...