Achieving C–N bond cleavage in dinuclear metal cyanide complexes†
Abstract
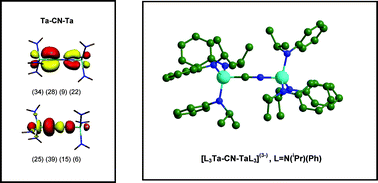
Cleavage of cyanide is more difficult to achieve compared to dinitrogen and carbon monoxide, even though these species contain triple bonds of greater strength. In this work, we have used computational methods to investigate thermodynamic and mechanistic aspects of the C–N bond cleavage process in [L3M–CN–M′L3] systems consisting of a central cyanide unit bound in an end-on fashion to two terminal metal tris-amide complexes. In these systems, [M] is a d3 transition metal from the 3d, 4d, 5d, or 6d series and groups 4 through 7, and [L] is either [NH2], [NMe2], [NiPrPh], or [NtBuAr]. A comparison of various models for the experimentally relevant [L3Mo–CN–MoL3] system has shown that while the C–N cleavage step appears to be an energetically favourable process, a large barrier exists for the dissociation of [L3Mo–CN–MoL3](−) into [L3Mo–C](−) and [N–MoL3], which possibly explains why C–N bond scission is not observed experimentally. The general structural, bonding, and thermochemical trends across the transition metal series investigated, indicate that the systems exhibiting the greatest degree of C–N

 Please wait while we load your content...
Please wait while we load your content...