Original palladium pincer complexes deriving from 1,3-bis(thiophosphinoyl)indene proligands: Csp3–H versus Csp2–H bond activation†
Abstract
A series of original ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)2]} (L =
S)2]} (L = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)2]} fragment is essentially governed by the conjugated and rigid nature of the dianionic pincer ligand, the nature of the coligand having little influence. The formation of the
S)2]} fragment is essentially governed by the conjugated and rigid nature of the dianionic pincer ligand, the nature of the coligand having little influence. The formation of the
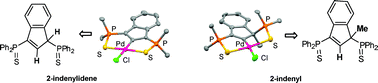
- This article is part of the themed collection: Pincers and other hemilabile ligands

 Please wait while we load your content...
Please wait while we load your content...