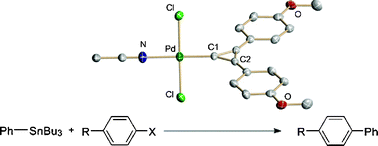
Reaction of [Pd(PPh3)4] with 1,1-dichloro-2,3-diarylcyclopropenes gives complexes of the type cis-[PdCl2(PPh3)(C3(Ar)2)] (Ar = Ph 5, Mes 6). Reaction of [Pd(dba)2] with 1,1-dichloro-2,3-diarylcyclopropenes in benzene gave the corresponding binuclear palladium complexes trans-[PdCl2(C3(Ar)2)]2 (Ar = Ph 7, p-(OMe)C6H48, p-(F)C6H49). Alternatively, when the reactions were performed in acetonitrile, the complexes trans-[PdCl2(NCMe)(C3(Ar)2)] (Ar = Ph 10, p-(OMe)C6H411 and p-(F)C6H4) 12) were isolated. Addition of phosphine ligands to the binuclear palladium complex 7 or acetonitrile adducts 11 and 12 gave complexes of the type cis-[PdCl2(PR3)(C3(Ar)2)] (Ar = Ph, R = Cy 13, Ar = p-(OMe)C6H4, R = Ph 14, Ar = p-(F)C6H4, R = Ph 15). Crystal structures of complexes 6·3.25CHCl3, 10, 11·H2O and 12–15 are reported. DFT calculations of complexes 10–12 indicate the barrier to rotation about the carbene-palladium bond is very low, suggesting limited double bond character in these species. Complexes 5–9 were tested for catalytic activity in C–C coupling (Mizoroki–Heck, Suzuki–Miyaura and, for the first time, Stille reactions) and C–N coupling (Buchwald–Hartwig amination) showing excellent conversion with moderate to high selectivity.

 Please wait while we load your content...
Please wait while we load your content...