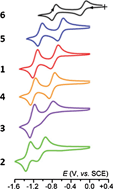
The icosahedral carboranes 1-C6F5-2-Ph-1,2-closo-C2B10H10 (1), 1-(4′-F3CC6H4)-2-Ph-1,2-closo-C2B10H10 (2), 1,2-(4′-F3CC6H4)2-1,2-closo-C2B10H10 (3), 1-(4′-H3CC6F4)-2-Ph-1,2-closo-C2B10H10 (4), 1-(4′-F3CC6F4)-2-Ph-1,2-closo-C2B10H10 (5), 1,2-(4′-F3CC6F4)2-1,2-closo-C2B10H10 (6), 1,7-(4′-F3CC6F4)2-1,7-closo-C2B10H10 (7) and 1,12-(4′-F3CC6F4)2-1,12-closo-C2B10H10 (8), with fluorinated aryl substituents on cage carbon atoms, have been prepared in good to high yields and characterised by microanalysis, 1H, 11B and 19F NMR spectroscopies, mass spectrometry, single-crystal X-ray diffraction and (spectro)electrochemistry. By analysis of <δ11B>, the weighted average 11B chemical shift, a ranking order for the orthocarboranes 1–6 is established based on the combined electron-withdrawing properties of the C-substituents, and is in perfect agreement with that established independently by electrochemical study. In a parallel computational study the effects of a wide range of different substituents on the redox properties of carboranes have been probed by comparison of ΔE values, where ΔE is the energy gap between the DFT-optimised [7,9-R2-7,9-nido-C2B10]2− anion and its DFT-optimised basket-shaped first oxidation product. The overall conclusion from the NMR spectroscopic, electrochemical and computational studies is that strongly electron withdrawing substituents significantly stabilise [7,9-nido-C2B10]2− dianions with respect to oxidation, and that the best practical substituent is 4-F3CC6F4. Thus attention focussed on the reduction of 1,2-(4′-F3CC6F4)2-1,2-closo-C2B10H10, compound 6. The sequence 6/[6]−/[6]2− appears reversible on the cyclic voltammetric timescale but on the longer timescale of macroelectrolysis the radical anion is only partially stable. EPR study of the electrogenerated monoanions from the ortho-carboranes 1–6 confirms the cage-centred nature of the redox processes. In contrast, the reduction of the meta- and para-carboranes 7 and 8, respectively, appears to be centred on the aromatic substituents, a conclusion supported by the results of DFT calculation of the LUMOs of compounds 6–8. Bulk 2-electron reduction of 6 affords a dianion which is remarkably stable to reoxidation, surviving for several hours in the open laboratory in the absence of halogenated solvents.

 Please wait while we load your content...
Please wait while we load your content...