On the mechanism of the reaction of white phosphorus with silylenes†
Abstract
Four different mechanisms and the effect of bulky groups were studied in the reaction of ({HC[CMeN(R)]2}Si, R = 2,6-diisopropylphenyl) with white phosphorus. The carefully tested B3LYP-D, ωB97X-D, and SOS-MP2 methods, by the CCSD(T) method, with cc-pVTZ basis set provided consonant results which support the reliability of our results and the applicability of these methods. The dispersion energy contribution to the total energy is estimated to about ∼70 kJ mol−1 which proves the essential role of dispersion corrected methods. Based on the computed results we suggest a new mechanism which is fully supported by the experimental conditions. The investigation of the bulky groups clearly demonstrates an internal catalytic feature which has an essential role in the reaction mechanism. Usually bulky substituents prevent or reduce the reactivity, in this case however, substituents promote the reaction.
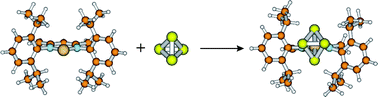

 Please wait while we load your content...
Please wait while we load your content...