Reactions of phosphinites with oxide surfaces: a new method for anchoring organic and organometallic complexes†
Abstract
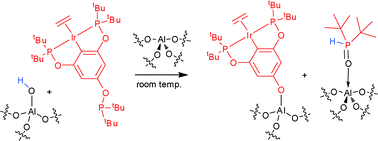
When a pincer-ligated iridium complex with a phosphinite substituent in the para-position of the aromatic backbone is immobilized on γ-alumina, it becomes a highly effective supported

 Please wait while we load your content...
Please wait while we load your content...