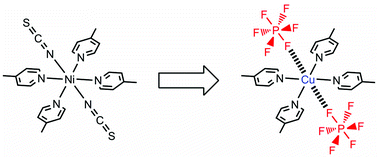
The quasi-Werner-type copper(II) complex, [Cu(PF6)2(4-mepy)4] (1), in which 4-mepy is the 4-methylpyridine ligand, has flexible and polar axial bonds of Cu–PF6. Flexibility of the Cu–PF6 bonds induces diverse and unprecedented guest-inclusion structures, such as {[Cu(PF6)2(4-mepy)4][Cu(PF6)(4-mepy)4(acetone)]·PF6·4acetone} (γ-1⊃2.5acetone), {[Cu(PF6)2(4-mepy)4][Cu(PF6)(4-mepy)4(2-butanone)]·PF6·3.5(2-butanone)} (γ-1⊃2.25(2-butanone)), {[Cu(PF6)2(4-mepy)4][Cu(PF6)(4-mepy)4(H2O)]·PF6·4benzene} (γ-1⊃0.5H2O·2benzene), and {[Cu(PF6)2(4-mepy)4]·2benzene} (γ-1⊃2benzene). Exposure of the dense form, α-1, to benzene vapor affords the benzene-inclusion complex {[Cu(PF6)2(4-mepy)4]·2benzene} (γ-1⊃2benzene), all benzene guests of which are easily removed by vacuum drying, reforming guest-free, dense α-1′ with smaller sized crystals than α-1. In contrast to α-1, which shows almost no CO2 adsorption, α-1′ adsorbs CO2 gas with structural transformations, this being the first example that exhibits adsorption of gas in a dense Werner-type complex and a drastic change in adsorption properties depending on the size of the crystals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...