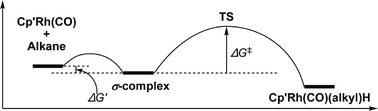
Fast time-resolved infrared spectroscopic measurements have allowed precise determination of the rate of C–H activation of alkanes by Cp′Rh(CO) {Cp′ = η5-C5H5 or η5-C5Me5; alkane = cyclopentane, cyclohexane and neopentane (Cp only)} in solution at room temperature and allowed the determination of how the change in rate of oxidative cleavage varies between complexes and alkanes. Density functional theory calculations on these complexes, transition states, and intermediates provide insight into the mechanism and barriers observed in the experimental results. Unlike our previous study of the linear alkanes, where activation occurred at the primary C–H bonds with a rate governed by a balance between these activations and hopping along the chain, the rate of C–H activation in cyclic alkanes is controlled mainly by the strength of the alkane binding. Although the reaction of CpRh(CO)(neopentane) to form CpRh(CO)(neopentyl)H clearly occurs at a primary C–H bond, the rate is much slower than the corresponding reactions with cyclic alkanes because of steric factors with this bulky alkane.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...