Binary and ternary uranium(vi) humate complexes studied by attenuated total reflection Fourier-transform infrared spectroscopy†
Abstract
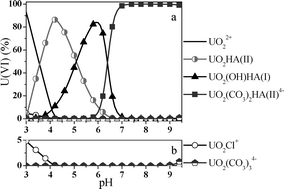
The complexation of U(VI) with humic acid (HA) in aqueous solution has been investigated at an ionic strength of 0.1 M (NaCl) in the pH range between pH 2 and 10 at different

 Please wait while we load your content...
Please wait while we load your content...