Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes†
Abstract
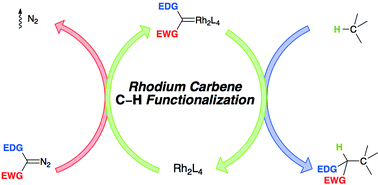
This tutorial review presents a description of the controlling elements of intermolecular C–H functionalization by means of C–H insertion by donor/acceptor
- This article is part of the themed collection: C–H Functionalisation in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...