BINEPINES: chiral binaphthalene-core monophosphepine ligands for multipurpose asymmetric catalysis
Abstract
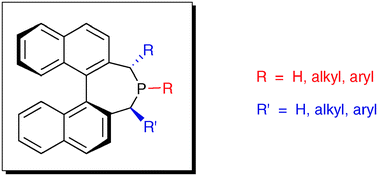
The atropisomeric structure of 4,5-dihydro-3H-dinaphtho[2,1-c;1′,2′-e]phosphepine is the common axially chiral scaffold of a library of monophosphine ligands nicknamed BINEPINES that have shown a quite remarkable stereoselection efficiency in a broad variety of enantioselective reactions involving the formation of new C–H or C–C or C–X bonds. In this critical review the properties and scope of this type of chiral ligands are illustrated (70 references).

 Please wait while we load your content...
Please wait while we load your content...