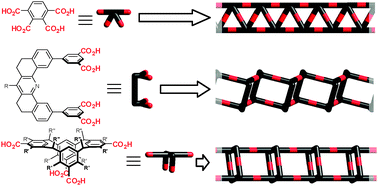
Hydrogen bonding is one of the most important non-covalent interactions in both biological (DNA, peptides, saccharides etc.) and artificial systems (various soft materials, host–guest architectures, molecular networks, etc.). Carboxylic acids are some of the most simple yet powerful hydrogen-bonding building blocks, that possess a particularly rich supramolecular chemistry. This tutorial review focuses on the structural diversity of supramolecular architectures accessible viahydrogen bonding of carboxylic acids, as observed both in single crystals using X-ray analysis and in monolayers on surfaces using scanning probe techniques. It provides a concise overview of the key concepts and principles of modern supramolecular design and is given in the form of case studies of finely selected literature examples, covering formation of macrocycles, chains, ladders, rotaxanes, catenanes, various 2D and 3D nets, host–guest systems and some applications thereof.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...