Structures and vibrational spectroscopy of partially reduced gas-phase cerium oxide clusters†
Abstract
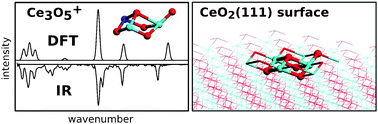
This work demonstrates that the most stable structures of even small gas-phase aggregates of cerium oxide with 2–5 cerium atoms show structural motifs reminiscent of the bulk ceria. This is different from main group and transition metal ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond show a characteristic absorption band between 800 and 840 cm−1. For some cluster sizes multiple isomers are observed. Their individual infrared signatures are identified by tuning their relative population through the choice of He, Ne or Ar messenger atoms. The present results allow us to benchmark different density functionals which yield different degrees of localization of unpaired electrons in Ce 4f states.
O bond show a characteristic absorption band between 800 and 840 cm−1. For some cluster sizes multiple isomers are observed. Their individual infrared signatures are identified by tuning their relative population through the choice of He, Ne or Ar messenger atoms. The present results allow us to benchmark different density functionals which yield different degrees of localization of unpaired electrons in Ce 4f states.

 Please wait while we load your content...
Please wait while we load your content...