Five potential reaction mechanisms, each leading to the formation of an α-O-4-linked coniferyl alcohol dimer, and one scheme leading to the formation of a recently proposed free-radical coniferyl alcohol trimer were assessed using density functional theory (DFT) calculations. These potential reaction mechanisms were evaluated using both the calculated Gibbs free energies, to predict the spontaneity of the constituent reactions, and the electron-density mapped Fukui function, to determine the most reactive sites of each intermediate species. The results indicate that each reaction in one of the six mechanisms is thermodynamically favorable to those in the other mechanisms; what is more, the Fukui function for each free radical intermediate corroborates with the thermochemical results for this mechanism. This mechanism proceeds via the formation of two distinct free-radical intermediates, which then react to produce the four α-O-4 stereoisomers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
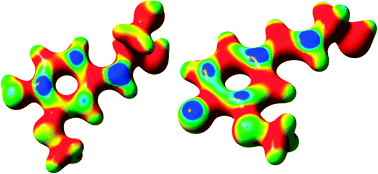

 Please wait while we load your content...
Please wait while we load your content...