Impact of tunneling on hydrogen-migration of the n-propylperoxy radical†
Abstract
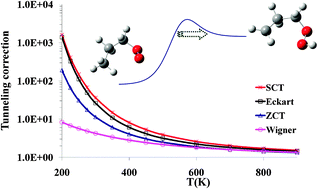
The kinetics of three unimolecular reactions of the n-propylperoxy radical were studied by canonical variational transition state theory and multidimensional small curvature tunneling (SCT). The reactions studied were 1,5 and 1,4 H-migration, and HO2 elimination. Benchmark calculations were carried out at the CCSD(T) level in order to determine which density functional to use for SCT calculations for each reaction. For 1,5 and 1,4 H-migration, and HO2 elimination, the M05-2X, B3LYP and B1B95 functionals, respectively, performed closest to the benchmark when coupled to the 6-311+G(2df,2p) basis set. The SCT tunneling corrections, κ(T), computed here were much larger than those calculated from the Wigner or zero-curvature tunneling treatments at low temperatures, but the asymmetric Eckart method works surprisingly well in these three reactions. Comparison of energy-dependent transmission coefficients, Γ(E), indicates that not only the magnitude, but also the sign, of the error in the Eckart approximation is a function of energy; therefore, the error introduced by using the Eckart approach depends strongly on the steady state energy distribution. These results may provide guidance for future studies of tunneling effects in reactions of other

 Please wait while we load your content...
Please wait while we load your content...