Recombination and chemical energy accommodation coefficients from chemical dynamics simulations: O/O2 mixtures reacting over a β-cristobalite (001) surface
Abstract
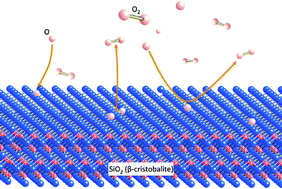
A microkinetic model is developed to study the reactivity of an O/O2 gas mixture over a β-cristobalite (001) surface. The thermal rate constants for the relevant elementary processes are either inferred from quasiclassical trajectory calculations or using some statistical approaches, resting on a recently developed interpolated multidimensional potential energy surface based on density functional theory. The kinetic model predicts a large molecular coverage at temperatures lower than 1000 K, in contrary to a large atomic coverage at higher temperatures. The computed atomic oxygen recombination coefficient, mainly involving atomic

 Please wait while we load your content...
Please wait while we load your content...