First-principles study of fluoroform adsorption on a hexagonal ice (0001) surface: weak hydrogen bonds—strong structural effects
Abstract
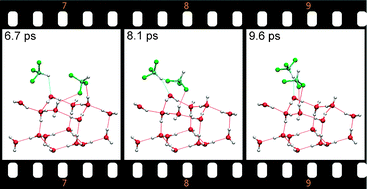
For isolated fluoroform (F3CH) molecules adsorbed on a hexagonal ice (0001) surface the properties of blue- and red-shifting hydrogen bonds were studied using static density functional theory (DFT) calculations and Car–Parrinello molecular dynamics (CP-MD) simulations. A systematic search by starting from many initial configurations was performed to determine the lowest-energy structures of F3CH on the ice surface, and for the optimized geometries the vibrational frequencies were calculated. The local minima structures are analyzed in terms of their coordination to the surface, with special focus on identifying blue-shifting hydrogen bonds via their spectroscopic signature of an increased frequency of the C–H fundamental stretching vibration. Subsequently, by CP-MD simulations the stability of the lowest-energy configurations at finite temperatures was verified and possible transformation pathways connecting the local minima structures were explored.
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...