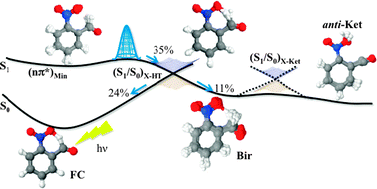
Ab initio surface-hopping dynamics calculations have been performed to simulate the intramolecular excited state hydrogen transfer dynamics of ortho-nitrobenzaldehyde (o-NBA) in the gas phase from the electronic S1 excited state. Upon UV excitation, the hydrogen is transferred from the aldehyde substituent to the nitro group, generating o-nitrosobenzoic acid through a ketene intermediate. The semiclassical propagations show that the deactivation from the S1 is ultrafast, in agreement with the experimental measurements, which detect the ketene in less than 400 fs. The trajectories show that the deactivation mechanism involves two different conical intersections. The first one, a planar configuration with the hydrogen partially transferred, is responsible for the branching between the formation of a biradical intermediate and the regeneration of the starting material. The conversion of the biradical to the ketene corresponds to the passage through a second intersection region in which the ketene group is formed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...