Abstract
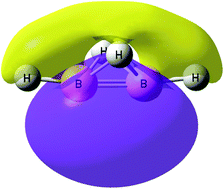
An ab initio study of an isomer of diborane(4) [B2H4] has been carried out at MP2/aug-cc-pVTZ to investigate the ground-state properties of this unusual molecule, a derivative of which has been described in the recent literature. The geometric, electronic and orbital characteristics of B2H4(4) have been analyzed using AIM, NBO, and ELF methodologies. A region with a high concentration of electron density is located near and along the B–B bond, on the opposite side of this bond relative to the bridging H atoms. This site serves as an electron-donor site to electrophiles, resulting in hydrogen-bonded complexes of B2H4 with
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...