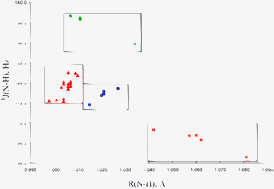
A systematic ab initio investigation has been carried out to determine the structures, binding energies, and spin–spin coupling constants of ternary complexes X:CNH:Z and corresponding binary complexes X:CNH and CNH:Z, for X, Z = CNH, FH, ClH, FCl, and HLi. The enhanced binding energies of ternary complexes X:CNH:Z for fixed X as a function of Z decrease in the same order as the binding energies of the binary complexes CNH:Z. In contrast, the enhanced binding energies of the ternary complexes for fixed Z as a function of X do not decrease in the same order as the binding energies of the binary complexes X:CNH, a consequence of the increased stabilities of ternary complexes FCl:CNH:Z due to very strong chlorine-shared halogen bonds. For complexes in which the X⋯CNH interaction is a D–H⋯C hydrogen bond for D–H the proton–donor group (N–H, F–H, or Cl–H), spin–spin coupling constants 1J(D–H) and 2hJ(D–C) in ternary complexes X:CNH:Z decrease in absolute value as the binding energies of binary complexes CNH:Z and the enhanced binding energies of the ternary complexes for fixed X as a function of Z also decrease. However, 2XJ(F–C) increases as the enhanced binding energies of the ternary complexes FCl:CNH:Z decrease, a consequence of the nature of the chlorine-shared halogen bond. The one-bond coupling constants 1J(N–H) for the CNH⋯Z interaction in ternary complexes vary significantly, depending on the nature of the X⋯CNH interaction. The largest values of 1J(N–H) are found for ternary complexes with FCl as X. Two-bond coupling constants 2hJ(N–A) for A the proton-acceptor atom of Z, and 2dJ(N–H) decrease in absolute value in the order of decreasing enhancement energies of ternary complexes X:CNH:Z for fixed Z as a function of X.
 Please wait while we load your content...
Please wait while we load your content...