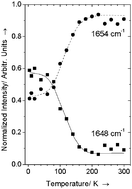
The properties of the intermolecular hydrogen bonds in the monoclinic (Form I) and the orthorhombic (Form II) polymorphs of paracetamol, C8H9NO2, have been studied by single crystal polarized Raman spectroscopy (40 to 3700 cm−1) in a wide temperature range (5 K < T < 300 K) in relation to the dynamics of methyl-groups of the two forms. A detailed analysis of the temperature dependence of the wavenumbers, bandwidths and integral intensities of the spectral bands has revealed an essential difference between the two polymorphs in the strength and ordering of OH⋯O and NH⋯O hydrogen bonds. The compression of intermolecular hydrogen bonds is interrelated with crystal packing and the dynamics of methyl-groups. On structural compression of the orthorhombic polymorph on cooling, a compromise is to be sought between the shortening of OH⋯O and NH⋯O bonds, attractive CH⋯O and repulsive CH⋯H contacts in the crystal structure. As a result of a steric conflict at temperatures below 100 K, N–H⋯O hydrogen bonds become significantly disordered, and an extended intramolecular transition from the conformation “staggered” with respect to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to the one “staggered” with respect to the NH bond is observed. In most of the studied crystals this transition was only about 60% complete even at 5 K, but in some of the crystals the orientation of all the methyl-groups became staggered with respect to the NH bond at low temperatures. This complete transition was coupled to a sharp shortening of the OH⋯O and NH⋯O hydrogen bonds at <100 K, the appearance of new additional positions of the protons in these H-bonds, and a slight strengthening of the C–H⋯O bonds formed by methyl-groups. The same conformational transition has been observed also in the monoclinic polymorph at T < 80 K. The crystal packing in Form I prevents the O–H⋯O hydrogen bonds from adopting the optimum geometry, and they are significantly disordered at all the temperatures, especially at ≤200 K. The packing of molecules in Form I is also not favourable to form C–H⋯O hydrogen bonds involving methyl-groups. One can conclude from the comparison of diffraction and spectroscopic data that the higher stability of Form I results not from a larger strength of individual OH⋯O and NH⋯O hydrogen bonds, but is a cumulative effect: all the hydrogen bonds together stabilize the structure of the monoclinic polymorph more than that of the orthorhombic polymorph.
O bond to the one “staggered” with respect to the NH bond is observed. In most of the studied crystals this transition was only about 60% complete even at 5 K, but in some of the crystals the orientation of all the methyl-groups became staggered with respect to the NH bond at low temperatures. This complete transition was coupled to a sharp shortening of the OH⋯O and NH⋯O hydrogen bonds at <100 K, the appearance of new additional positions of the protons in these H-bonds, and a slight strengthening of the C–H⋯O bonds formed by methyl-groups. The same conformational transition has been observed also in the monoclinic polymorph at T < 80 K. The crystal packing in Form I prevents the O–H⋯O hydrogen bonds from adopting the optimum geometry, and they are significantly disordered at all the temperatures, especially at ≤200 K. The packing of molecules in Form I is also not favourable to form C–H⋯O hydrogen bonds involving methyl-groups. One can conclude from the comparison of diffraction and spectroscopic data that the higher stability of Form I results not from a larger strength of individual OH⋯O and NH⋯O hydrogen bonds, but is a cumulative effect: all the hydrogen bonds together stabilize the structure of the monoclinic polymorph more than that of the orthorhombic polymorph.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to the one “staggered” with respect to the NH bond is observed. In most of the studied crystals this transition was only about 60% complete even at 5 K, but in some of the crystals the orientation of all the
O bond to the one “staggered” with respect to the NH bond is observed. In most of the studied crystals this transition was only about 60% complete even at 5 K, but in some of the crystals the orientation of all the 
 Please wait while we load your content...
Please wait while we load your content...