Facile solid-phase synthesis of the diammoniate of diborane and its thermal decomposition behavior†
Abstract
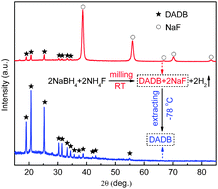
The recent mechanistic finding of the hydrogen release pathways from ammonia borane (AB) has sparked new interest in the chemistry and properties of the diammoniate of

 Please wait while we load your content...
Please wait while we load your content...