Unfolding dynamics of cytochrome c revealed by single-molecule and ensemble-averaged spectroscopy†
Abstract
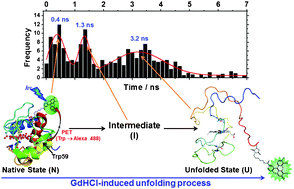
Denaturant-induced conformational change of yeast iso-1-cytochrome c (Cytc) has been comprehensively investigated in the single-molecule and bulk phases. By fluorescence-quenching experiments with
- This article is part of the themed collection: Biophysics and biophysical chemistry in PCCP

 Please wait while we load your content...
Please wait while we load your content...