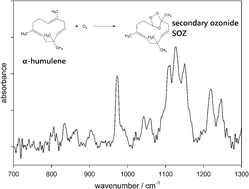
α-Humulene contains three double bonds (DB), and after ozonolysis of the first DB the first-generation products are still reactive towards O3 and produce second- and third-generation products. The primary aim of this study consisted of identifying the products of the three generations, focusing on the carboxylic acids, which are known to have a high aerosol formation potential. The experiments were performed in a 570 litre spherical glass reactor at 295 K and 730 Torr. Initial mixing ratios were 260–2090 ppb for O3 and 250–600 ppb for α-humulene in synthetic air. Reactants and gas-phase products were measured by in situFTIR spectroscopy. Particulate products were sampled on Teflon filters, extracted with methanol and analyzed by LC-MS/MS-TOF. Using cyclohexane (10–100 ppm) as an OH-radical scavenger and by monitoring the yield of cyclohexanone by PTR-MS, an OH-yield of (10.5 ± 0.7)% was determined for the ozonolysis of the first DB, and (12.9 ± 0.7)% of the first-generation products. The rate constant of the reaction of O3 with α-humulene is known as k0 = 1.17 × 10−14 cm3 molecule−1 s−1 [Y. Shu and R. Atkinson, Int. J. Chem. Kinet., 1994, 26, 1193–1205]. The reaction rate constants of O3 with the first-generation products and the second-generation products were, respectively, determined as k1 = (3.6 ± 0.9) × 10−16 and k2 = (3.0 ± 0.7) × 10−17 cm3 molecule−1 s−1 by Facsimile-simulation of the observed ozone decay by FTIR. A total of 37 compounds in the aerosol phase and 5 products in the gas phase were tentatively identified: 25 compounds of the first-generation products contained C13–C15 species, 9 compounds of the second-generation products contained C8–C11 species, whereas 8 compounds of the third-generation products contained C4–C6 species. The products of all three generations consisted of a variety of dicarboxylic-, hydroxy-oxocarboxylic- and oxo-carboxylic acids. The formation mechanisms of some of the products are discussed. The residual FTIR spectra indicate the formation of secondary ozonides (SOZ) in the gas phase, which are formed by the intramolecular reaction of the Criegee moiety with the carbonyl endgroup. These SOZ revealed to be stable over several hours and its formation was shown not to be affected by the addition of Criegee-radical scavengers such as HCOOH or H2O. This suggests that in the ozonolysis of α-humulene at atmospheric pressures the POZ will decompose rapidly, and that a large fraction of the formed exited Criegee Intermediate will be stabilized to form stable SOZ, while the formation of OH-radicals via the hydroperoxide channel will be a minor process.

 Please wait while we load your content...
Please wait while we load your content...