Nature and strength of C–H⋯O interactions involving formyl hydrogen atoms: computational and experimental studies of small aldehydes†
Abstract
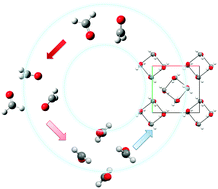
Structural and electronic properties of C–H⋯O contacts in compounds containing a
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...