The first study on enhanced photoresponsivity of ZnO–TiO2 nanocomposite thin films by anodic polarization
Abstract
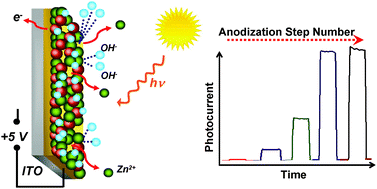
The physical and photoelectrochemical properties of the anodized ZnO–TiO2 thin films were investigated in this study. Impedance spectroscopy revealed a decrease in charge transfer resistance and Tafel plots determined enhancement of about 200 times in the exchange current (i0) after

 Please wait while we load your content...
Please wait while we load your content...