Phase transition induced improvement in H2 desorption kinetics: the case of the high-temperature form of Y(BH4)3†‡
Abstract
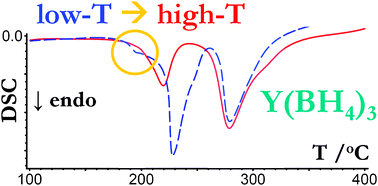
The high-temperature (HT) phase of Y(BH4)3 has been prepared by heating of the as mechanochemically synthesised low-temperature (LT) phase of Y(BH4)3 to 194–216 °C and subsequent rapid cooling to ambient temperature. Although the differences in the crystal structure and

 Please wait while we load your content...
Please wait while we load your content...