Low-temperature formation of cubic β-PbF2: precursor-based synthesis and first-principles phase stability study†
Abstract
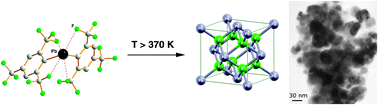
A precursor-based approach to the cubic β-phase of PbF2 was developed and allowed the preparation of this high-temperature phase well below the temperature for transition from the orthorhombic α- to the cubic β-phase. The formation of β-PbF2 from the molecular precursors Pb[Se(C6H2(CF3)3)]2 and Pb(C6H2(CF3)3)2 is facilitated by the presence of several short Pb⋯F contacts in these molecules. The cubic form of PbF2 was obtained as macroscopic crystals as well as nanoparticulate powder. Its formation at relatively low temperature suggested a theoretical re-investigation of the phase stabilities of the two polymorphs. The theoretical results from the Kohn–Sham density functional theory indicate that the energy content for the β-phase is slightly lower than the one for the α-phase, by 0.5–1.7 kJ mol−1 depending on the density functional used (zero-point vibrational energy correction included).

 Please wait while we load your content...
Please wait while we load your content...