Stability conditions for density functional reactivity theory: An interpretation of the total local hardness
Abstract
The second-order Taylor series expansions commonly used in the density functional chemical reactivity theory are used to define local stability conditions for electronic states. Systems which satisfy these conditions are stable to infinitesimal perturbations due to approaching chemical reagents. The basic formalism considered here supersedes previous variational approaches to chemical reactivity theory like the electrophilicity, potentialphilicity, and chargephilicity. The total local hardness emerges naturally in this analysis, and can be clearly interpreted. When the total local hardness is small, the system is relatively insensitive to perturbations. Furthermore, minus the total local hardness is an energetically favorable perturbation of the external potential.
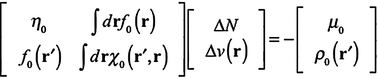

 Please wait while we load your content...
Please wait while we load your content...