Quantum-chemical predictions of redox potentials of carbamates in methanol†
Abstract
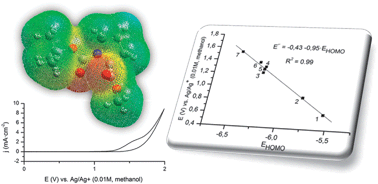
Redox potentials for two stepwise anodic oxidations of a series of carbamates in methanolic solution have been calculated using ab initio and DFT quantum mechanical methods. Hartree–Fock method and Density Functional Theory at the B3LYP level, together with 6-31G(d), 6-31G(d,p) and 6-311++G(2df,2p) basis sets have been used for the calculation. The Polarizable Continuum Model (

 Please wait while we load your content...
Please wait while we load your content...