How do electron localization functions describe π-electron delocalization?†
Abstract
Scalar fields provide an intuitive picture of chemical bonding. In particular, the electron
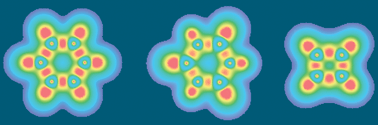
- This article is part of the themed collection: Aromaticity, electron delocalisation, and related molecular properties

 Please wait while we load your content...
Please wait while we load your content...