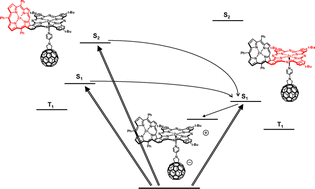
In the current work, we report on the synthesis and photophysical features of supramolecular hybrid systems that are based on newly fused porphyrin–phthalocyanine (P–Pc) conjugates and a pyridylfullerene. The ZnP–ZnPc conjugate was synthesized in three steps starting with a Diels–Alder reaction between β-vinylporphyrin and fumaronitrile. The resulting mixture of isomeric adducts was then dehydrogenated to yield the corresponding benzo[b]porphyrin-21,22-dicarbonitrile. In the final step, cyclotetramerization with 4-tert-butylphthalonitrile, in the presence of zinc acetate, afforded the bis-metalated conjugate. Selective demetallation of ZnP led to the H2P–ZnPc conjugate. For both conjugates steric hindrance is the inception to a bent configuration, which does, however, not preclude enlargement of the π-conjugated system, that is, the porphyrins and the phthalocyanines. The two conjugates coordinate N-(4-pyridyl)fullero[c]pyrrolidine giving rise to the corresponding supramolecular porphyrin–phthalocyanine–fullerene systems. Photophysical measurements corroborate a sequential deactivation in the excited state, namely an initial intramolecular energy transfer from ZnP or H2P to ZnPc followed by an intramolecular charge transfer to yield ZnP–(ZnPc)˙+–(C60)˙− and H2P–(ZnPc)˙+–(C60)˙−, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...