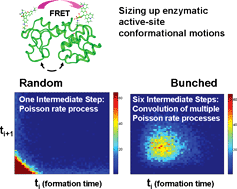
In this perspective, we focus our discussion on how the single-molecule spectroscopy and statistical analysis are able to reveal enzyme hidden properties, taking the study of T4 lysozyme as an example. Protein conformational fluctuations and dynamics play a crucial role in biomolecular functions, such as in enzymatic reactions. Single-molecule spectroscopy is a powerful approach to analyze protein conformational dynamics under physiological conditions, providing dynamic perspectives on a molecular-level understanding of protein structure–function mechanisms. Using single-molecule fluorescence spectroscopy, we have probed T4 lysozyme conformational motions under the hydrolysis reaction of a polysaccharide of E. coli B cell walls by monitoring the fluorescence resonant energy transfer (FRET) between a donor–acceptor probe pair tethered to T4 lysozyme domains involving open–close hinge-bending motions. Based on the single-molecule spectroscopic results, molecular dynamics simulation, a random walk model analysis, and a novel 2D statistical correlation analysis, we have revealed a time bunching effect in protein conformational motion dynamics that is critical to enzymatic functions. Bunching effect implies that conformational motion times tend to bunch in a finite and narrow time window. We show that convoluted multiple Poisson rate processes give rise to the bunching effect in the enzymatic reaction dynamics. Evidently, the bunching effect is likely common in protein conformational dynamics involving in conformation-gated protein functions. In this perspective, we will also discuss a new approach of 2D regional correlation analysis capable of analyzing fluctuation dynamics of complex multiple correlated and anti-correlated fluctuations under a non-correlated noise background. Using this new method, we are able to map out any defined segments along the fluctuation trajectories and determine whether they are correlated, anti-correlated, or non-correlated; after which, a cross correlation analysis can be applied for each specific segment to obtain a detailed fluctuation dynamics analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...