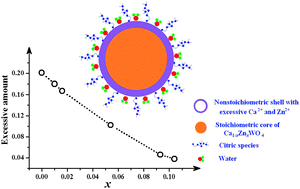
Chemical composition directly determines the structure and properties of almost all bulk inorganic solids, which are however popularly dismissed in the literature as a cause of property changes when studying multi-component oxide nanostructures by solution chemistries. The current work focuses on this subject through a systematic case study on CaWO4 nanocrystals. CaWO4 nanocrystals were prepared using room-temperature solution chemistry, in which a capping agent of citric acid was employed for kinetic grain size control. Sample characterizations by a set of techniques indicated that 5–7 nm CaWO4 was obtained at room temperature, showing a pure-phase of tetrahedral scheelite structure. The molar ratio of Ca2+ to W6+ was found to be 1.2 : 1, apparently deviating from the unity expected for the stoichiometric CaWO4. Such nonstoichiometry was further modulated via iso-valent incorporation of smaller Zn2+ to the Ca2+-sites in CaWO4. It is found that with increasing the Zn2+ content, there appeared transformation from high to low nonstoichiometry, though a pure scheelite-typed structure was retained. Such a nonstoichiometry was primarily represented by excessive cations like Zn2+ and/or Ca2+ within the surface disorder layers, which in turn showed a great impact on the structure and properties as demonstrated by a lattice contraction, band-gap narrowing, luminescence quenching, as well as improved conductivity. The property changes were rationalized in terms of surface structural disorder, electro-negativity discrepancy, and effective activation on the mobile protons. Consequently, systematic control over the non-stoichiometry for single-phase multi-component oxide nanostructures by solution chemistry is proven fundamentally important, which may help to achieve quantitatively the structure–property relationship for materials design and performance optimization.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...