Demystifying the solvatochromic reversal in Brooker’s merocyanine dye†
Abstract
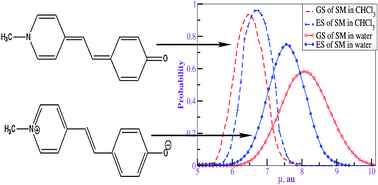
Based on hybrid QM/MM simulation techniques, we rationalize the spectacular solvatochromic reversal behavior observed for a stilbazolium merocyanine (SM) called Brooker's merocyanine

 Please wait while we load your content...
Please wait while we load your content...