Origin of improved visible photocatalytic activity of nitrogen/hydrogen codoped cubic In2O3: first-principles calculations†
Abstract
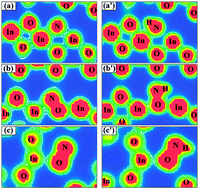
We have employed DFT calculations to carry out an accurate analysis of the effect of N- and NH-doping on the visible photocatalytic activity in the cubic In2O3. In the substitutional N-doped In2O3, the 2p impurity states of N induce a red shift in the optical absorption, while in the interstitial N-doping the red shift is dominantly caused by the localized π antibonding states of NO. When a H atom is accompanied by a N impurity in the lattice, the H atom acts as a charge donor and compensates the hole state created by N-doping, thus the energy level of the impurity states is reduced. As a result, the mixing of impurity states and the valence band is enhanced. At the same

 Please wait while we load your content...
Please wait while we load your content...