Energetics and structure of single Ti defects and their influence on the decomposition of NaAlH4
Abstract
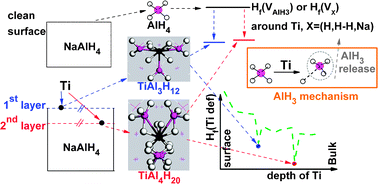
The energetics and structure of various types of single extrinsic Ti defects in NaAlH4 bulk and (001) slab at the hydriding/dehydriding critical point environment were studied systematically. It is found that the most favorable situation is Ti substituting Al at the subsurface (TiAl(2nd)), which has the highest coordination number for extrinsic Ti ions. The most stable Ti defect in the 1st layer is located at the Al rich interstitial site, namely Tii(1st), accompanied with remarkable strength of Ti–H/Al bond and local geometry deformation at the 1st layer around Ti. Deeper insight of the formation mechanism of Ti defects is obtained by dividing the formation enthalpy of Ti defects into three terms, which are contributed from the cost of removing a substituted host atom if necessary, the cost of structure deformation, and the gain of bonding between Ti and its surrounding ions in the formation of the defects. This associates the formation energy directly with the local structure of Ti defects. For the first time, we adopt Hf(H), Hf(H–H), Hf(AlH3) and Hf(Na) to discuss the hydrogen release ability of the Ti doped NaAlH4. We find that TiAl4H20 and TiAl3H12 complexes are formed around TiAl(2nd) and Tii(1st) respectively, which significantly promotes the dehydriding ability of NaAlH4. What is more, the

 Please wait while we load your content...
Please wait while we load your content...