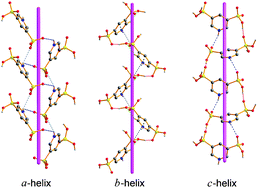
Three pyridylphosphonic acids, 3-pyridylphosphonic acid 1, 5-(dihydroxyphosphoryl)nicotinic acid 2, and 3,5-pyridinediyldiphosphonic acid 3, were synthesized and structurally investigated by solid state FT-IR and single crystal X-ray diffraction methods. All compounds appear in zwitterionic forms in the solid state with a proton transferred from the phosphonic group toward the pyridine N-atom. Strong hydrogen-bond interactions O–H⋯O and N–H⋯O organize the molecules of the compounds into polar three-dimensional networks. The crystal structures are additionally stabilized via weaker C–H⋯O hydrogen bonds and π⋯π or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π interactions. Powder second harmonic generation and solution NMR spectra were measured as well. The NMR spectra revealed that the double bonds of the functional groups (P
O⋯π interactions. Powder second harmonic generation and solution NMR spectra were measured as well. The NMR spectra revealed that the double bonds of the functional groups (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), although differently oriented versus the Npy atom, are coplanar with the aromatic rings in all stable, low energy conformers of compounds 1–3 in solution. In crystals, however, the P
O), although differently oriented versus the Npy atom, are coplanar with the aromatic rings in all stable, low energy conformers of compounds 1–3 in solution. In crystals, however, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are tilted toward the ring by 10.77(7)° in 1, 70.42(8)° in 2 and by 43.97(7)° and 6.56(8)° in 3. All three compounds exhibit moderate powder SHG efficiencies compared to that of urea.
O bonds are tilted toward the ring by 10.77(7)° in 1, 70.42(8)° in 2 and by 43.97(7)° and 6.56(8)° in 3. All three compounds exhibit moderate powder SHG efficiencies compared to that of urea.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π interactions. Powder second harmonic generation and
O⋯π interactions. Powder second harmonic generation and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), although differently oriented versus the Npy atom, are coplanar with the aromatic rings in all stable, low energy conformers of compounds 1–3 in solution. In crystals, however, the P
O), although differently oriented versus the Npy atom, are coplanar with the aromatic rings in all stable, low energy conformers of compounds 1–3 in solution. In crystals, however, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are tilted toward the ring by 10.77(7)° in 1, 70.42(8)° in 2 and by 43.97(7)° and 6.56(8)° in 3. All three compounds exhibit moderate powder SHG efficiencies compared to that of
O bonds are tilted toward the ring by 10.77(7)° in 1, 70.42(8)° in 2 and by 43.97(7)° and 6.56(8)° in 3. All three compounds exhibit moderate powder SHG efficiencies compared to that of 

 Please wait while we load your content...
Please wait while we load your content...