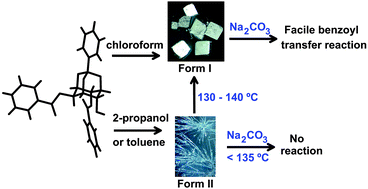
Racemic 2,6-di-O-benzoyl-myo-inositol 1,3,5-orthobenzoate (rac-3), which usually crystallizes in a monoclinic lattice (Form I, P21/c) from common organic solvents, when crystallized from 2-propanol yielded concomitantly, thin whisker like crystals (Form II, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) with inclusion of 2-propanol and water molecules, along with the Form I crystals. Thin fibre-like crystals were also obtained on crystallization from toluene, with inclusion of toluene and water in the crystal lattice (Form III). The Form II and Form III crystals could be converted into the Form I crystals thermally via melt crystallization. Form I crystals exhibit a facile transesterification reaction, but the solvated crystals are unreactive under the same conditions until their transformation to the reactive form. The reactivity patterns of the Form I and Form II crystals correlate well with the molecular organization in them. Since reactivity of the crystals of rac-3 depends on the solvent and the method of crystallization, and the thermal transition of one crystal form to the other, these phase changes can be used as a switch to control the benzoyl transfer reactivity of the constituent molecules in crystals.
) with inclusion of 2-propanol and water molecules, along with the Form I crystals. Thin fibre-like crystals were also obtained on crystallization from toluene, with inclusion of toluene and water in the crystal lattice (Form III). The Form II and Form III crystals could be converted into the Form I crystals thermally via melt crystallization. Form I crystals exhibit a facile transesterification reaction, but the solvated crystals are unreactive under the same conditions until their transformation to the reactive form. The reactivity patterns of the Form I and Form II crystals correlate well with the molecular organization in them. Since reactivity of the crystals of rac-3 depends on the solvent and the method of crystallization, and the thermal transition of one crystal form to the other, these phase changes can be used as a switch to control the benzoyl transfer reactivity of the constituent molecules in crystals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) with inclusion of
) with inclusion of 

 Please wait while we load your content...
Please wait while we load your content...