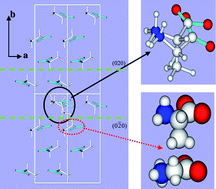
Molecular modelling has been applied to study the mechanism through which L-alanine, acting as an impurity, disrupts the growth and modifies the morphology of α-glycine crystals. L-alanine was selected for study because of its structural similarity to glycine differing only by the substitution of a methyl group for a hydrogen atom. An approach employing an atom-atom formalism has been extended to model the effects of the chiral molecule, L-alanine, when it becomes incorporated as an impurity in α-glycine (achiral) crystals. A number of cases are treated which provide a quantitative measure of the changes in lattice-site energy within the host α-glycine lattice accompanying incorporation of an L-alanine molecule. Both the conformation of the L-alanine molecule and the possibility of creating a site vacancy in the host lattice are taken into account. Slice and attachment energies are calculated for the significant growth forms {020}, {011} and {110} in the presence of L-alanine. A new approach is described which employs a weighted average of attachment energies calculated for the pure host, and host modified by additive, to predict crystal morphology and its dependence on the proportion of lattice sites at which host molecules are replaced by additive molecules. The results show that whereas L-alanine is likely to incorporate both at the growing (020) (which has the pro-chiral R hydrogen atom, bonded to the α carbon, orientated normal to the surface) and (0![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) crystal surface of α-glycine (which has the pro-chiral S hydrogen, atom bonded to the α carbon, orientated normal to the surface), the reduction in the rate of growth perpendicular to the (0
0) crystal surface of α-glycine (which has the pro-chiral S hydrogen, atom bonded to the α carbon, orientated normal to the surface), the reduction in the rate of growth perpendicular to the (0![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) surface, ascribed from the reduction in the associated relative attachment-energy, is greater. The crystal morphologies predicted are found to be in general agreement with experimental observations reported in the literature.
0) surface, ascribed from the reduction in the associated relative attachment-energy, is greater. The crystal morphologies predicted are found to be in general agreement with experimental observations reported in the literature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) crystal surface of α-glycine (which has the pro-chiral S hydrogen, atom bonded to the α carbon, orientated normal to the surface), the reduction in the rate of growth perpendicular to the (0
0) crystal surface of α-glycine (which has the pro-chiral S hydrogen, atom bonded to the α carbon, orientated normal to the surface), the reduction in the rate of growth perpendicular to the (0![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) surface, ascribed from the reduction in the associated relative attachment-energy, is greater. The crystal morphologies predicted are found to be in general agreement with experimental observations reported in the literature.
0) surface, ascribed from the reduction in the associated relative attachment-energy, is greater. The crystal morphologies predicted are found to be in general agreement with experimental observations reported in the literature.

 Please wait while we load your content...
Please wait while we load your content...