Two metal–organic frameworks (MOFs) based on Zn(II)-squarate bridges associated with non-covalent and covalent bonds of dpe ligands, [Zn(dpe)(C4O4)(H2O)2]n (1), and {[Zn(Hdpe)(C4O4)0.5(H2O)3][Zn(C4O4)2(H2O)2]}n (2) (C4O42− (squarate) = dianion of 3,4-dihydroxycyclobut-3-ene-1,2-dione (H2C4O4); dpe = 1,2-bis(4-pyridyl)ethane), have been synthesized and characterized by spectroscopic analysis and single-crystal X-ray diffraction studies. Compound 1 is a two-dimensional (2D) metal–organic framework (MOF), being constructed via the crosslinkage of one-dimensional (1D) [Zn(μ1,3-squarate)] chains and 1D sinusoidal-like [Zn(gauche-dpe)] chains. Compound 2 is a 2D tri-layered composite network composed of one cationic polythreading [Zn(Hdpe)(C4O4)0.5(H2O)3]2+ MOF and two anionic [Zn(C4O4)2(H2O)2]2− MOFs with the penetration of the monoprotonated anti-form Hdpe+ ligands dangling above and below the 2D Zn(II)-(μ1,2,3,4-squarate) layer of cationic [Zn(Hdpe)(C4O4)0.5(H2O)3]2+ MOF into the windows of two 2D Zn(II)-(μ1,3-squarate) layers of anionic [Zn(C4O4)2(H2O)2]2− MOFs. Both of their 2D MOFs in compounds 1 and 2 are then extended to 3D supramolecular architectures via the intermolecular hydrogen bonding interaction among the squarate, protonated dpe ligands and water molecules.
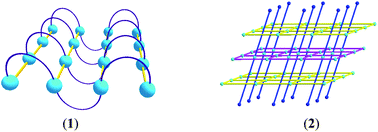
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
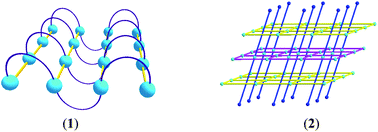

 Please wait while we load your content...
Please wait while we load your content...