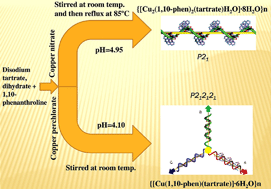
Two different chiral metal–organic frameworks (CMOF), namely, {[Cu2(1,10-phen)2(tartrate)H2O]·8H2O}n (1) and {[Cu(1,10-phen)(H2tartrate)H2O]·6H2O}n (2) [1,10-phen = 1,10-phenanthroline] have been synthesized with an alteration in the pH of the reaction media. Reflux at 85 °C, complex 1 (pH = 4.95) crystallizes in P21, while simple stirring at room temperature, complex 2 (pH = 4.10) crystallizes in P212121 space group. When the solid-state structure of 1 is compared with complex 2, interesting changes in the supramolecular self-assembly are noticed. CMOF 1 features a 1D supramolecular coordination polymer, in stark contrast, CMOF 2 features 1D coordination polymer. We reveal one helicalassembly in 1 whereas three helicalassembly in 2. The CMOFs exhibit solid-state blue luminescence at room temperature and non-linear optical (NLO) activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...