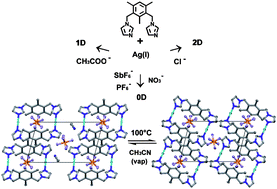
A series of silver(I) complexes with the ditopic ligand 1,3-bis(imidazol-1-ylmethyl)-2,4,6-trimethylbenzene (bitmb) and SbF6−, PF6−, NO3−, CH3COO−, Cl− anions was prepared. The complexes were obtained in the same 1 : 1 metal to ligand molar ratio and reflect the influence of the counterions on the final structural arrangement. X-Ray diffraction revealed formation of similar discrete, dinuclear, metallocyclic species, for [Ag2(bitmb)2](SbF6)2·2CH3CN (1), [Ag2(bitmb)2](PF6)2·2CH3CN (2) and [Ag2(bitmb)2](NO3)2 (3) with di-coordinated silver ions. The solvent molecules present in the structures (1 and 2) are not strongly bonded and are easy to remove, resulting in a reversible single-crystal-to-single-crystal transformation (2 ↔ 2a). The remaining two compounds [Ag(bitmb)CH3COO]n (4) and [Ag(bitmb)Cl]n (5) show polymeric structures with 1D and 2D ordering, respectively. In those complexes, counterions participate in the coordination sphere of the metal centre, yielding tri- and tetra-coordinated silver(I) compounds, correspondingly.

 Please wait while we load your content...
Please wait while we load your content...