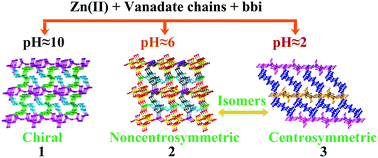
Three organic–inorganic hybrid compounds have been designed and synthesized based on different vanadate chains, Zn(II) ions and flexible ligands at different pH values under hydrothermal conditions, namely, [Zn2(bbi)2(V3O9)(OH)]·H2O (1), [Zn2(bbi)2(V4O12)] (2), and [Zn(bbi)(V2O6)] (3), where bbi is 1,1′-(1,4-butanediyl)bis(imidazole). In 1, helical chains [(V3O9)3−]∞ were connected by [Zn2(OH)]3+ dimers giving rise to a 2D chiral layer which was further bridged via bbi ligands to construct a 3D POM-based chiral framework. There are two opposite helical chains [(V4O12)4−]∞ in 2. The [(V4O12)4−]∞ chains with the same chirality were connected each other by Zn2 cations to generate a 2D chiral layer, respectively. The adjacent chiral layers were linked by Zn1 ions to generate a 3D non-centrosymmetric framework with large channel encased by bbi. Similarly, the [(V2O6)2−]∞ chains with the same chirality in 3 were connected by zinc atoms to form a 2D chiral layer which was further linked through bbi to generate a 3D centrosymmetric network. Compounds 2 and 3 are supramolecular isomers. With adjusting the pH value of reaction mixture, we have achieved three new hybrids crystallized from chiral, non-centrosymmetric to centrosymmetric space group.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...