Stereoselective synthesis of complex polycyclic aziridines: use of the Brønsted acid-catalyzed aza-Darzens reaction to prepare an orthogonally protected mitomycin C intermediate with maximal convergency†
Abstract
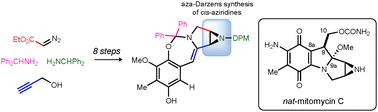
A concise synthesis of a highly functionalized intermediate lacking only C10 of the mitomycin backbone is described. The key to this development is the Brønsted acid-catalyzed aza-Darzens reaction used to forge the cis-aziridine. Additionally an oxidative ketalization fortuitously occurs during the

 Please wait while we load your content...
Please wait while we load your content...